AnIML Workshop "Putting AnIML to Work"
at PittCon 2009, March 9, 2009 in Chicago, IL.
Abstract:The Analytical Information Markup Language is (AnIML) is being jointly developed by ASTM Subcommittee E13.15 on Analytical Data and IUPAC Subcommittee on Electronic Data Standards for organizing, interchanging, and archiving most analytical chemistry result data and metadata. The initial ASTM documentary standards for AnIML are slated to come out in 2009. These will include standard specifications detailing the AnIML format and standard guides for using AnIML. Standards documents covering initial individual techniques (UV/VIS, IR, MS, 1D-NMR, chromatography and their combinations) are expected to follow. Even though AnIML is not yet complete, several vendor and user institutions have been testing and evaluating it to develop use cases and to demonstrate how AnIML might fit into their operations. Following an update on the current status of AnIML, this workshop will highlight some of these pre-standard activities that have been critical to the development of the AnIML standard, will detail the open source activities and licensing issues surrounding AnIML, and will conclude with a demonstration of several different AnIML generic data viewers. Additional information can be found on the AnIML website: animl.sourceforge.net.
Agenda
- 1:00 pm Introductory Remarks - GARY KRAMER, NIST
- 1:35 pm Status of AnIML MAREN FIEGE, Waters GmbH (Paper 710-1)
- 2:05 pm Use Cases for the Analytical Information Markup Language (AnIML) BURKHARD A SCHAEFER, BSSN Software (Paper 710-2)
- 2:35 pm Open Source Licensing of AnIML JAMIE MCQUAY, Scimatic Software (Paper 710-3)
- 3:20 pm An AnIML Deployment in Preclinical Development for Compliant Chromatography Long Term Storage and Archiving ANTHONY DAVIES ALIS Ltd/ALIS GmbH (Paper 710-4)
- 3:50 pm Native AnIML LCMS Processing and Viewing MAREN FIEGE, Waters GmbH (Paper 710-5)
- 4:20 pm Playing with the AnIMLs: Demonstrations of AnIML Generic Viewers GARY KRAMER, NIST (Paper 710-6)
ABSTRACTS
Paper 710-1: STATUS OF ANIML
Maren Fiege, Waters GmbH, Europaallee 27-29, Frechen, NRW 50226, Germany
Analytical instruments and software today produce huge amounts of data - most of it in different proprietary formats. Exchanging and comparing data from different sources can thus become a challenge, as can the need for long term availability of data when applications are retired and formats become obsolete.
Human-readable standard data formats are the preferred way to address these challenges as they are publicly available and well-documented and long-term stable.
After the previous efforts of ANDI and JCAMP, the Analytical Information Markup Language (AnIML) is a recent, ongoing joint project of the ASTM Subcommittee E13.15 on Analytical Data and the IUPAC Subcommittee on Electronic Data Standards. Representatives from industry, vendors, users, and academia are working together on developing AnIML as a new data standard format flexible enough to accommodate all kinds of analytical data from any technique.
This talk will give a short introduction on what AnIML is, what it is not, and what its goals are, as well as a high-level overview on its structure. Additionally, information will be provided on the current status of the AnIML project and any ongoing activities.
(Presentation not currently available)
Paper 710-2: USE CASES FOR THE ANALYTICAL INFORMATION MARKUP LANGUAGE (ANIML)
Burkhard A. Schaefer, BSSN Software, Postfach 411145, Mainz 55068, Germany
The Analytical Information Markup Language (AnIML) is a standardization effort of the E13.15 Sub-Committee of the American Society for Testing and Materials (ASTM). AnIML defines an XML-based format for documentation of laboratory experiments and their results. It is suitable for a wide range of analytical measurement techniques.
To achieve this, AnIML provides a generic data container that permits the storage of arbitrary analytical data. The concept of Technique Definitions permits the formal specification of constraints for using this data container. This way, a definition can prescribe how the data for specific measurement techniques should be captured in the data file.
This presentation will examine a number of use cases where AnIML can help facilitate processes in today's laboratory environments.
We will begin with a simple example which describes the data from a single analytical instrument. This example will then be expanded to reflect more complex real-life situations: Multiple analytical techniques will be added to the experiment. Multiple users then interact with the data, including workflow management and approval stages. In this context, the application of digital signatures and audit trails is discussed. Later, the data are prepared for long-term archival.
Other use cases discussed will include instrument connectivity, data exchange, and integration with other laboratory software components.
Click to download presentation
Paper 710-3: OPEN SOURCE LICENSING OF ANIML
Jamie McQuay, Scimatic Software, 436-550 Front St. West, Toronto, Ontario M5V-3N5, Canada
The ASTM Subcommittee E13.15 is currently developing the Analytical Information Markup Language (AnIML). This standard will provide a defined data format, making the contents of a file accessible to any AnIML aware software application.
In the age of software interoperability, the success of a new data standard is dependent on the ability of the data to be accessible to everyone.
The AnIML standard, once completed, will be licensed with an open source license. Using an open source license approach will ensure that the format remains consistent and accessible to all interested parties (instrument vendors, software developers, researchers etc...)
This talk will give an overview of the benefits of the open source license that will be applied to the AnIML standard.
Click to download presentation
Paper 710-4: AN ANIML DEPLOYMENT IN PRECLINICAL DEVELOPMENT FOR COMPLIANT CHROMATOGRAPHY LONG TERM STORAGE AND ARCHIVING
Antony N. Davies, ALIS Ltd/ALIS GmbH, Lemberger Feld 22, Dortmund 44229, Germany
In recent years ALIS Ltd has been involved in the development of the IUPAC/NIST AnIML standard and has also been employed to assist in a deployment of a beta version of the standard within the pharmaceutical industry. The talk will highlight why the draft AnIML data standard was selected and deployed as the file format of choice for compliant data storage and archiving at a major global pharmaceutical company. The arguments behind the decisions taken to go down this route are reflected in the Vendor selection process. Major milestones, problems encountered and solutions developed over the last six years will be discussed and finally issues around ambiguous regulatory positions and shifting goals will also be explored.
(Presentation not currently available)
Paper 710-5: NATIVE ANIML LCMS PROCESSING AND VIEWING
Mark F. Bean, GlaxoSmithKline, Up12-210, 1250 Collegeville Rd, Collegeville, PA 19426
Adventures in representing LCMS data in XML will be recounted by an experienced C#.Net developer and LCMS practitioner, active in the ASTM working party since its inception. Two years ago we showed a sophisticated, vendor-independent LCMS data viewer using AnIML converted from Microsoft typed datasets. This year, drawing on progress in the realization of LC, UV, and MS technique definitions for AnIML, we will explore using AnIML as the native file format. This will include, if possible, conversion of entire LCMS data files, creation of LCMS reports, and representation of graphical results in AnIML. The talk will include discussion of various AnIML XML files, as well as a dynamic vendor-independent LCMS data viewer capable of re-extracting spectra or extracted ion chromatograms on demand from the original files. We will discuss challenges in the creation and parsing of these XML files.
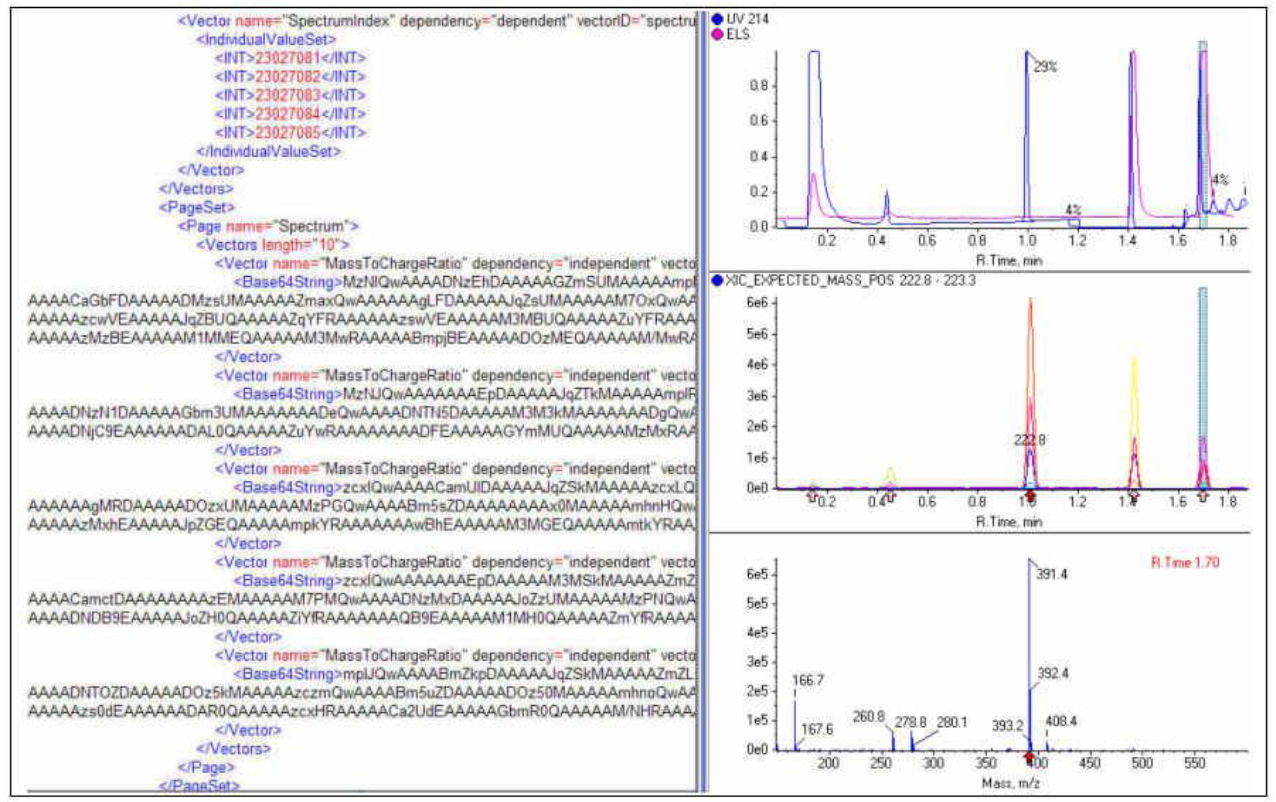
Click to download presentation
Paper 710-6: PLAYING WITH THE ANIMLS: DEMONSTRATIONS OF ANIML GENERIC VIEWERS
Gary W. Kramer, NIST, 100 Bureau Drive, Bldg. 227; Rm. - A-161; Ms 8310, Gaithersburg, MD 20899-8310
The Analytical Information Markup Language (AnIML) is being created as a mechanism for the interchange and archiving of analytical result information using the eXtensible Markup Language (XML). AnIML provides standardized structure and organization for the data and metadata that result from an analysis. One consequence of having a highly structured, self-describing, XML-based data format is that this enables the creation of generic viewing programs. In its simplest form a generic viewer provides a screen image of spectral, chromatographic, or other data stored in an AnIML file in a form useful for the analyst. More complex viewers can deal with multidimensional data and provide manipulation and processing capabilities. Generic data viewers will be important to folks such as analytical laboratory managers and principal investigators who need to review data from many different instruments, but who cannot afford to invest the time and resources for learning the multiple, specific, machine-dependent software programs commonly used to process and display analytical data from multiple instruments.
This presentation will begin with a general description of how data from diverse analytical experiments are stored in AnIML files and conclude with a demonstration of some generic AnIML viewers.